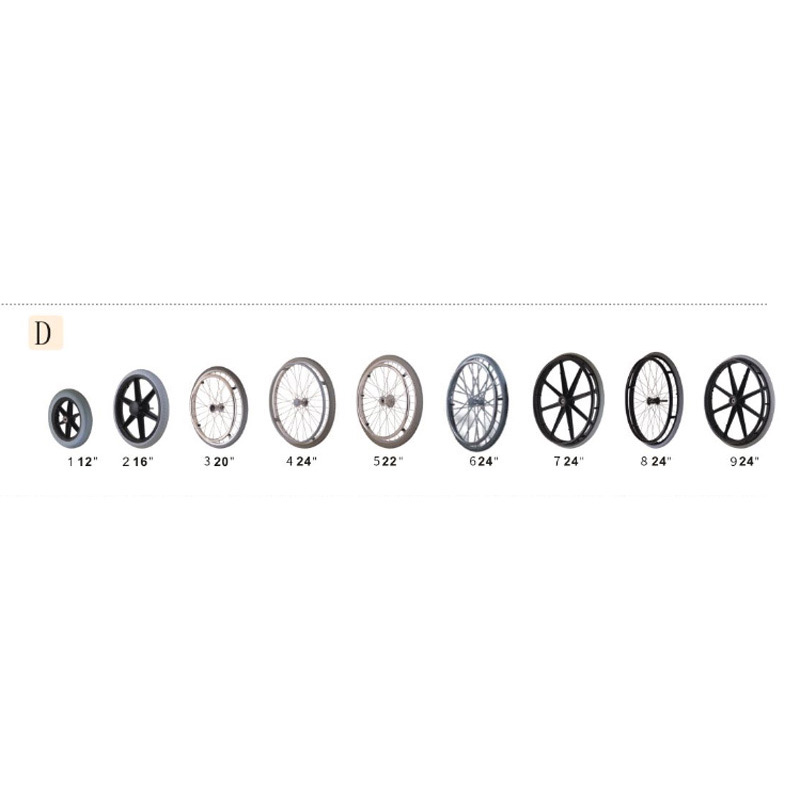
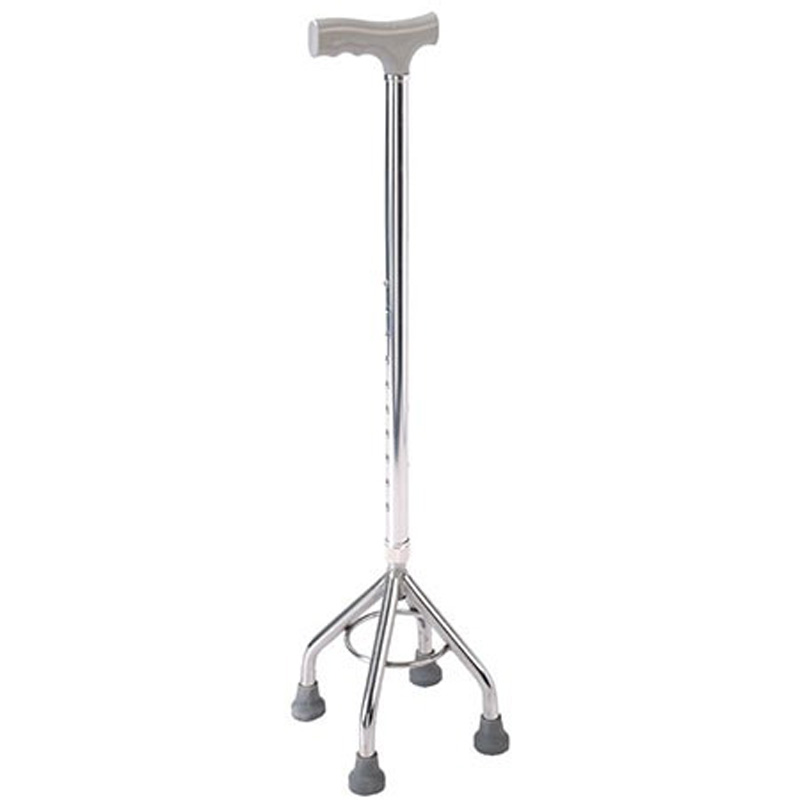
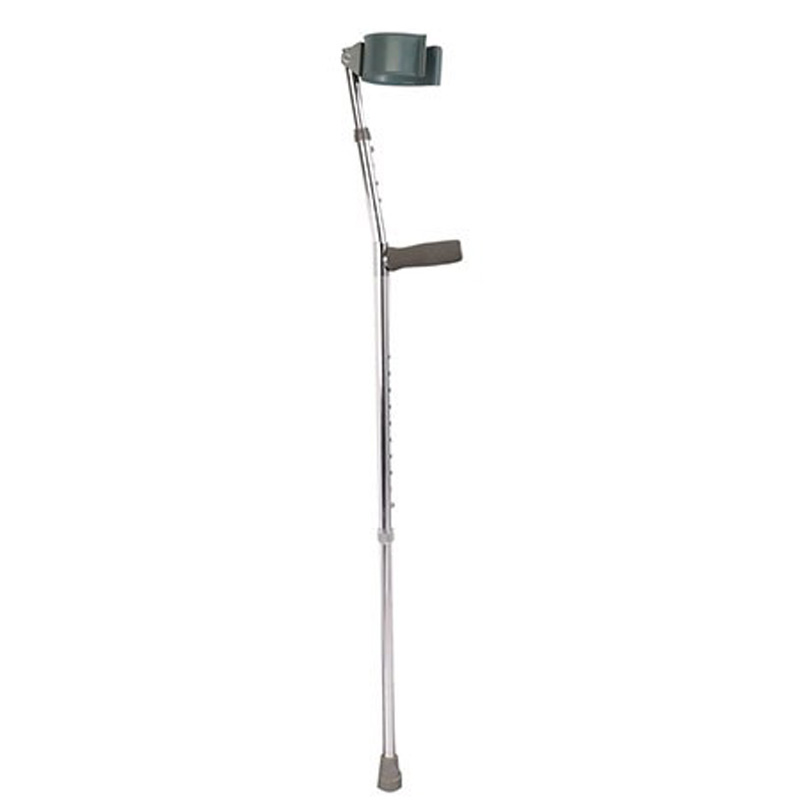
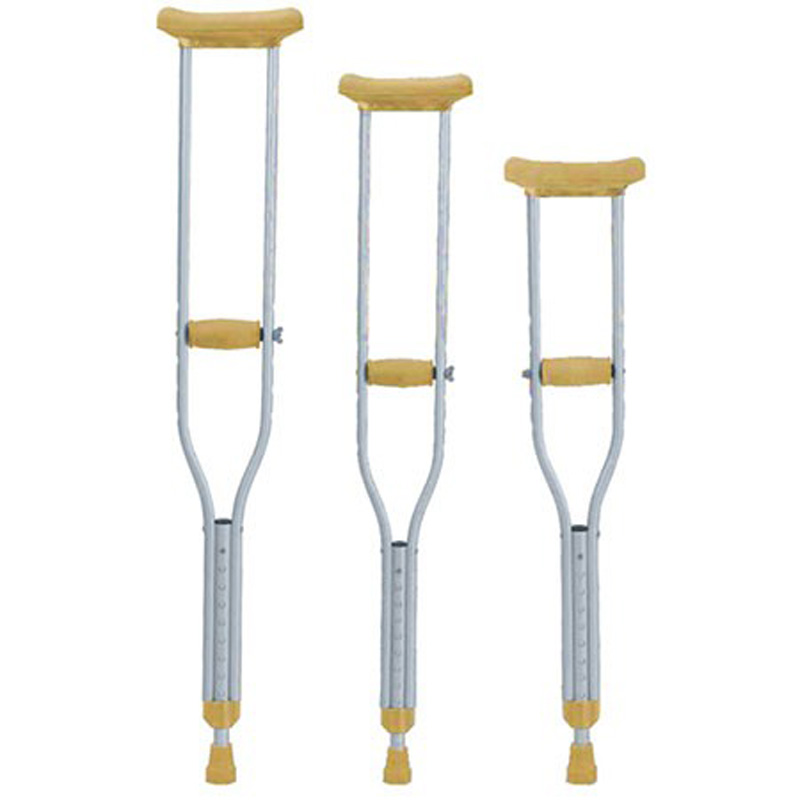
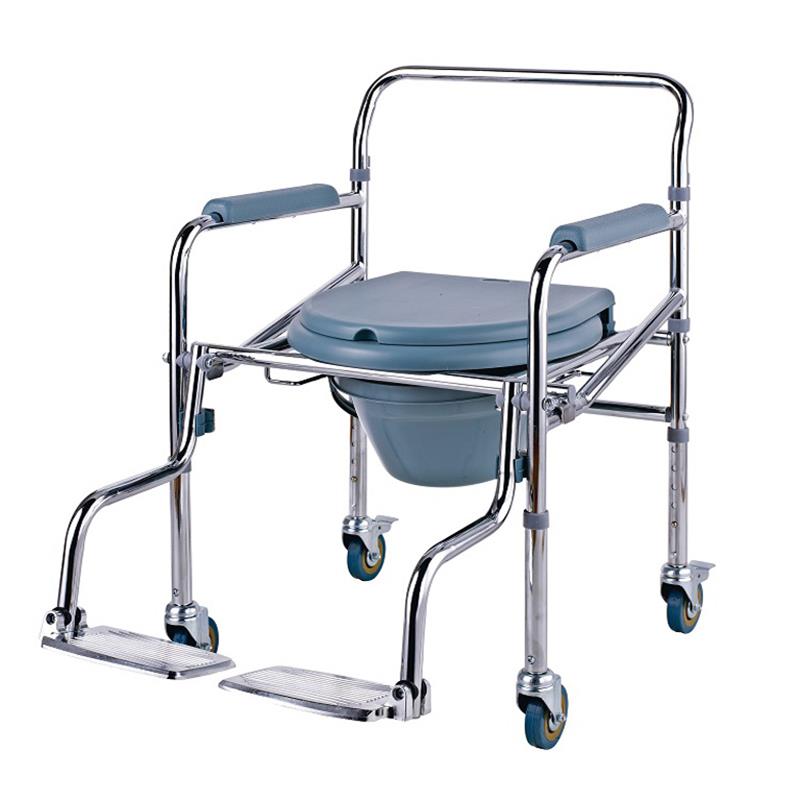
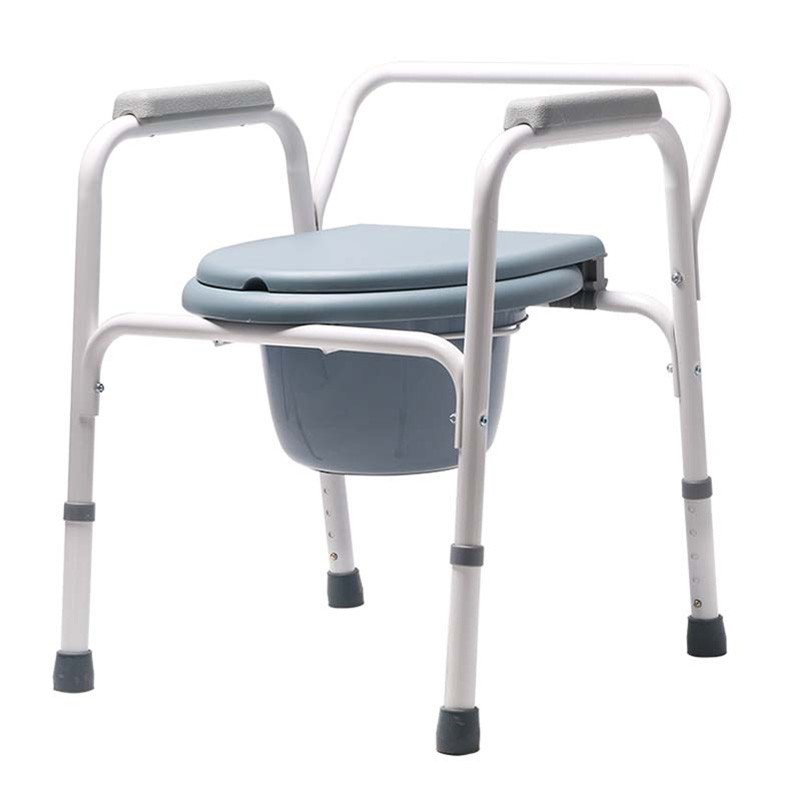
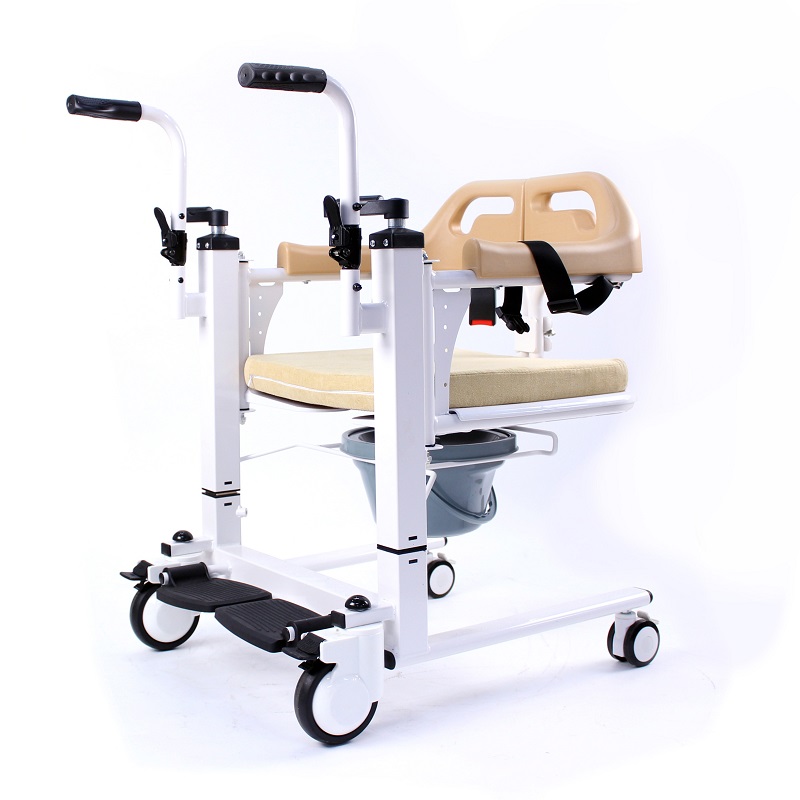
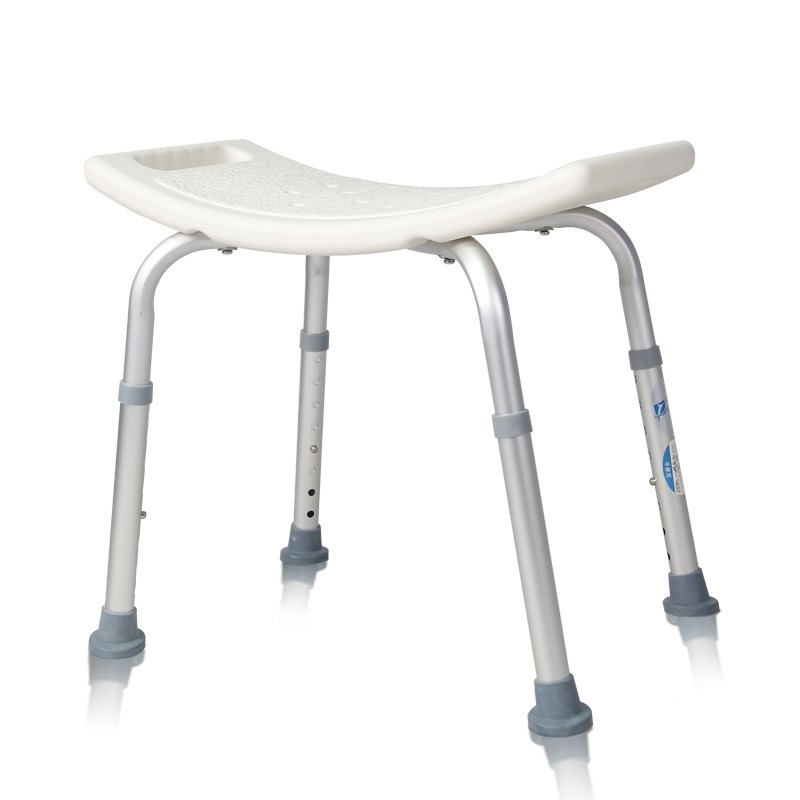
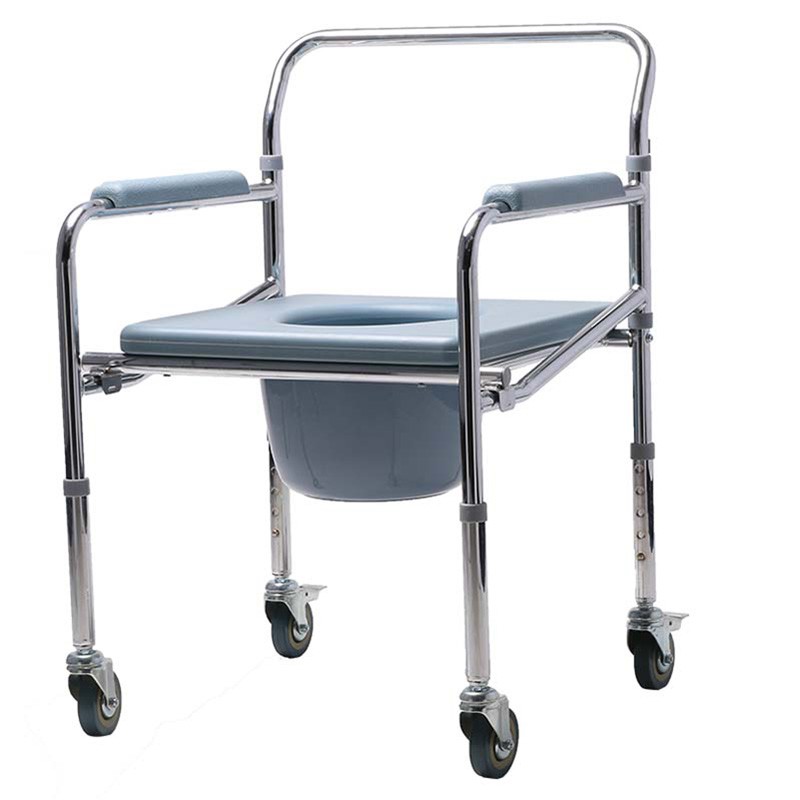
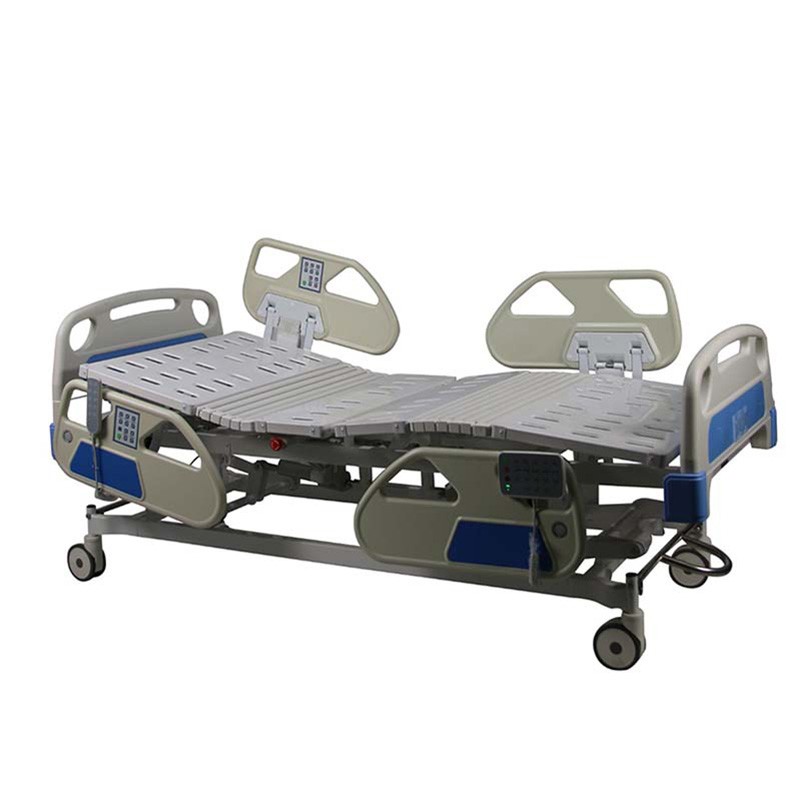
ISO13485 is a quality management system for medical devices to meet regulatory requirements. Since medical devices are special products for saving lives, preventing diseases and curing illnesses, it is not enough to regulate them only according to the general requirements of ISO 9000 standard. For this reason, ISO organisation promulgated ISO13485, which puts forward special requirements on the quality management system of medical device manufacturers, and plays a very good role in promoting the safety and efficacy of the quality of medical devices. It has played a good role in promoting the quality of medical devices.
To be certified to the ISO 13485 standard, companies must comply with the requirements set out in the ISO 13485 standard. Companies use the standard to demonstrate their ability to consistently provide products and services that meet customer and regulatory requirements and to demonstrate continuous improvement.